Resonance structure of phenol

1.Impressions of Phenol Resonance Structure
1. The wide application of phenol
phenol, the "all-ronged" in the field of organic chemistry, shines in many fields. In the field of medicine, it is the key raw material for the synthesis of aspirin and other important drugs, escorting human health; in the chemical industry, it is the "main force" in the production of phenolic resin, helping the development of material science; in daily life, it is also used as Disinfectant raw materials guard our health and safety. With such a wide range of applications, phenol has become one of the hot spots in organic chemistry research.
2. Lead to the concept of resonance structure
in the wonderful world of organic chemistry, resonant structures are a unique theoretical model. It is like a magic key that can help us open the door to the mystery of the electron distribution inside the molecule. For phenol, the resonance structure is the key to understand its properties.
3. Propose the purpose of the study
in this paper, we will deeply analyze the resonance structure of phenol, explore the internal relationship between it and the properties of phenol, and provide a theoretical basis for us to better understand and utilize phenol.
2.Formation, Characteristics, and Effects of Phenol Resonance Structure
1. Formation mechanism of resonance structure of phenol
(1) Basis of molecular structure
phenol consists of a benzene ring and a hydroxyl group. The benzene ring has a unique large & pi; bond structure in which electrons can move freely throughout the ring. Oxygen atoms, on the other hand, have lone pairs of electrons, which are like "elves" hidden in the dark, waiting for a wonderful reaction with the benzene ring.
(2) Electron delocalized process
when the phenol molecule is formed, the lone pair electrons of the oxygen atom interact strongly with the large & pi; bond of the benzene ring. The electrons are no longer confined to a particular atom, but are redistributed within the molecule, forming multiple resonance limit formulas. This is like a wonderful dance performance, electronic free shuttle in the molecules, performing a unique "electronic dance".
(3) Superposition of resonance limit type
these resonance limit formulas do not exist independently, but are superimposed on each other through resonance. They are like a group of "team members" working together to describe the true electron cloud distribution of the phenol molecule. It is this superposition that gives the phenol molecule its unique properties.
2. Phenol resonance structure characteristics
(1) High density of adjacent para electron cloud
due to the resonance effect, the electron cloud density of the adjacent and para positions on the benzene ring increases significantly. From the schematic diagram, the electron clouds in these two positions are like "lit" bulbs, which are particularly eye-catching. This high electron cloud density makes the ortho and para sites more active in chemical reactions.
(2) the delocalization of negative charge
negative charge on the benzene ring occurred delocalized phenomenon, like a group of free birds, flying freely in the "sky" of the benzene ring. This delocality reduces the molecular energy and makes the structure more stable, which lays the foundation for the chemical properties of phenol.
3. Effect of resonance structure on the properties of phenol
(1) Reactivity
electrophilic substitution reaction: The high density of the ortho-para electron cloud makes phenol more prone to electrophilic substitution reaction. For example, in nitration, sulfonation and other reactions, the reactants will preferentially attack the ortho and para positions to generate corresponding products, and the products are mainly concentrated in the ortho and para positions.
Reaction selectivity: In some reactions, phenol exhibits a specific reaction selectivity due to the resonance structure. Just like a picky eater, choosing only the "food" he likes, this selectivity provides more possibilities for organic synthesis.
(2) Acidity
the resonance structure makes it easier for the phenolic hydroxyl hydrogen to dissociate, thereby enhancing the acidity of the phenol. Compared with alcohols, phenol is significantly more acidic, like a more "pungent" molecule, more likely to release hydrogen ions.
(3) Stability
the resonance stabilization energy reduces the energy of the phenol molecule, making its structure more stable and less prone to decomposition or change. This makes phenol more reliable in storage and use, and provides a guarantee for its wide application.
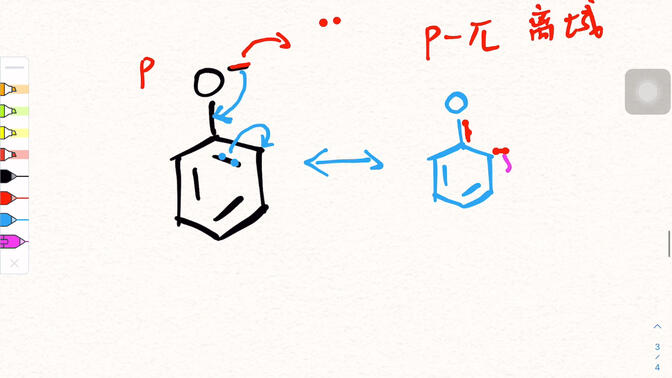
4.Draw the resonance structure of phenol
The resonance structure of phenol can be presented through a simple schematic diagram.
The basic structure of phenol is a hydroxyl group (- OH) attached to the benzene ring.
A resonance limit equation is the original structure of phenol, in which oxygen atom lone pair electrons do not participate in delocalization;
In another limit equation, the lone pair electron of the oxygen atom interacts with the large π - bond of the benzene ring, forming a partial double bond characteristic between the oxygen atom and the benzene ring. At the same time, the density of adjacent and para electron clouds on the benzene ring increases, and the direction of electron movement is indicated by arrows. Multiple limit equations are superimposed to describe the true electron cloud distribution of phenol.
3.The manifestation of resonance structure in phenol reaction
1. Specific response cases
the reaction of phenol with bromine water is a classic case. When bromine water is added dropwise to the phenol solution, a white precipitate is immediately produced, which is tribromophenol.
2. Combined with resonance structure analysis
from the point of view of the resonance structure, the bromine atom is more likely to attack these two positions due to the high density of the ortho-and para-electron clouds of phenol. During the reaction, bromine atoms gradually replace the hydrogen atoms on the benzene ring, and finally a white precipitate of tribromophenol is generated. This process clearly demonstrates the important role of the resonance structure in explaining the chemical reaction of phenol.
3. Emphasize the role of resonant structures
through this case, we have a deeper understanding of the resonance structure is not only a theoretical concept, but also the key to understand the nature of the chemical reaction of phenol. It is like a silent conductor, guiding the chemical reaction.
4.The value and prospect of studying the resonance structure of phenol
the resonance structure makes phenol have unique reactivity, acidity and stability, which further determines the application of phenol in various fields. In the future, we can combine advanced computational chemistry methods and experimental techniques to further study the resonance structure of phenol. This will help us to understand the nature and behavior of phenol more comprehensively, provide more theoretical support and practical guidance for the development of organic chemistry, and let the "generalist" of phenol play a greater role in more fields.

